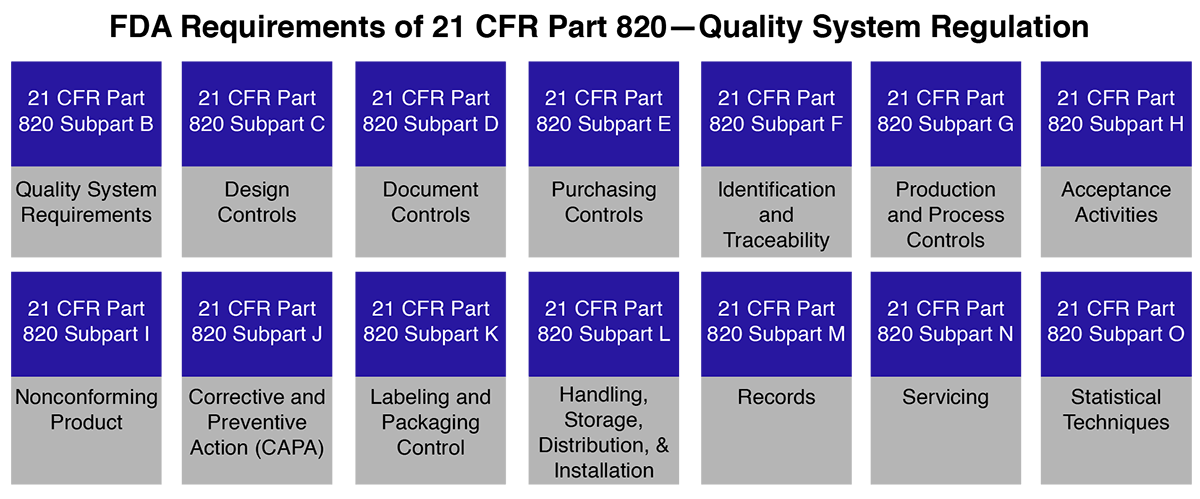
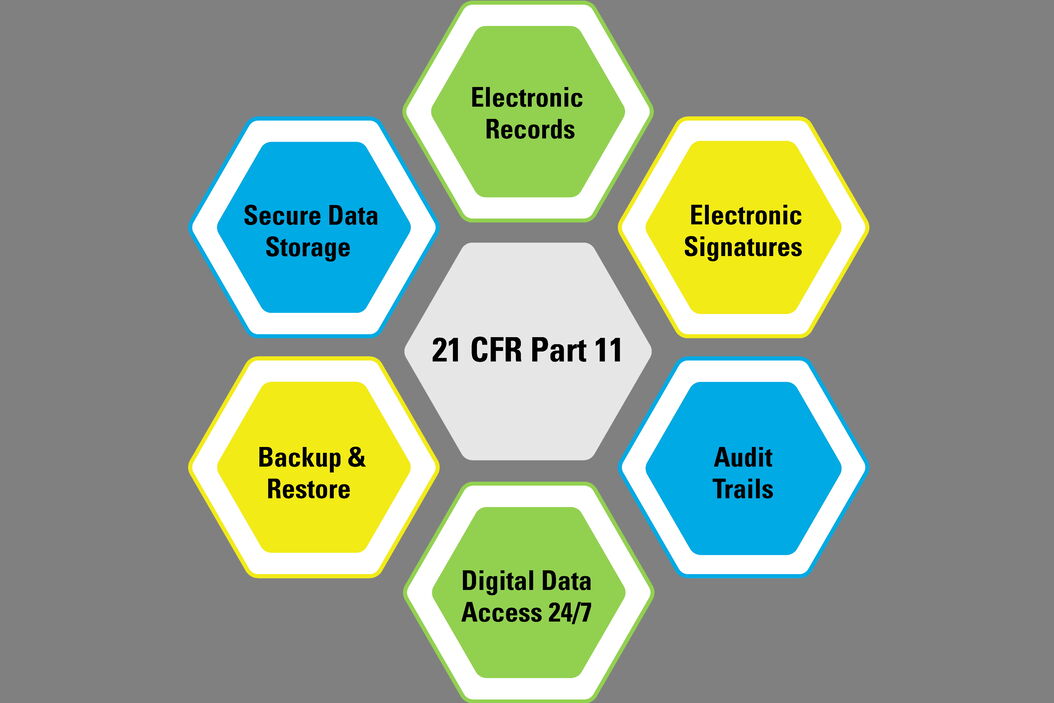
Code of Federal Regulations, Title 21 Food and Drugs 1300-End, Revised as of April 1, 2021 - 9781636718415

The Complete Code of Federal Regulations, Title 21, Food And Drugs, FDA Regulations, 2016 - Kindle edition by United States Government. Professional & Technical Kindle eBooks @ Amazon.com.

Amazon | CODE OF FEDERAL REGULATIONS TITLE 21 Food And Drugs BUDGET EDITION 2018 PARTS 1-99: CFR TITLE 21 | FEDERAL REGISTER, OFFICE OF THE | Law

FDA CFR Part 11 Compliance | Security Assessment | Compliance Services | Certification & Attestation | DIY Platform

Food and Drug Administration Title 21 CFR Part 11 Technology Company Title 21 of the Code of Federal Regulations png download - 1300*1300 - Free Transparent Food And Drug Administration png Download. - CleanPNG / KissPNG

CODE OF FEDERAL REGULATIONS TITLE 21 Food And Drugs BUDGET EDITION 2018 PARTS 1-99: CFR TITLE 21 eBook : FEDERAL REGISTER, OFFICE OF THE: Amazon.in: Kindle Store
.jpg)